Background:The vitamin K-dependent coagulation factor protein Z ( PROZ) is canonically considered to have anticoagulant effects through its negative regulation of factor Xa. However, the true role of protein Z in coagulation is controversial, with conflicting data on the impact of plasma protein Z levels across a range of thrombotic phenotypes. In particular, higher circulating protein Z concentrations have consistently been associated with an elevated risk of acute ischemic stroke, but there is little explanation for this apparent paradox. Because studies of plasma protein levels are especially prone to confounding by environmental factors, a large-scale, adjusted analysis examining the thrombosis risk associated with germline loss-of-function variants in PROZ would represent a novel approach with far greater capacity to address causality.
Aims:Perform a large-scale genomic association study to examine the risk associated with functional variants in PROZ across four thrombotic phenotypes.
Methods:Using paired clinical and whole exome sequencing data for 416,767 participants in the UK Biobank (UKBB), we identified individuals with rare (MAF<0.1%), in silico predicted function-altering (∆F) variants in PROZ. Using Firth's logistic regression and controlling for age, sex, ancestry, and other known risk factors for thrombosis, we assessed the association between ∆F variants in PROZ and venous thromboembolism (VTE), non-cardioembolic ischemic stroke (NCEIS), peripheral arterial disease (PAD), myocardial infarction (MI), and coagulopathic bleeding. Additionally, we evaluated differences in the levels of 1,472 plasma proteins (Olink Explore 1536 proteomics panel) between carriers and non-carriers of qualifying PROZ variants in a subset of 48,892 UKBB participants.
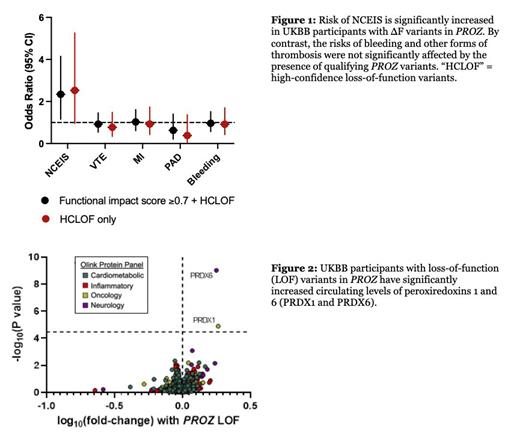
Results: Rare ∆F variants in PROZ were identified in 463 UKBB participants (99.14% heterozygous). The presence of ∆F variants was associated with significantly increased risk of NCEIS (OR=2.34, 95% CI: 1.16-4.15, P=0.02). By contrast, no significant association was seen between PROZ ∆F variants and VTE (OR=0.92, 95% CI: 0.54-1.46, P=0.75), MI (OR=1.03, 95% CI: 0.61-1.61, P=0.93), PAD (OR=0.63, 95% CI: 0.21-1.40, P=0.29), or coagulopathic bleeding (OR=0.96, 95% CI: 0.57-1.52, P=0.88) ( Figure 1). Restricting the analysis to high-confidence loss of function variants (i.e., nonsense, frameshift, and essential splice site mutations) increased the effect size estimate for NCEIS (OR=2.53, 95% CI: 0.96-5.26, P=0.058) without meaningfully changing our findings in the other thrombotic or bleeding phenotypes. Plasma proteomic analysis revealed that carriers of a PROZ ∆F variant had significantly elevated levels of two proteins that have previously been found to mediate brain inflammation after acute ischemic stroke, peroxiredoxin 1 (PRDX1, fold-change = 1.83, P=1.3 x 10 -5) and peroxiredoxin 6 (PRDX6, fold-change = 1.78, P=9.6 x 10- 10) ( Figure 2).
Conclusions:In this dataset of over 400,000 participants, we found that rare, germline loss-of-function variants in PROZ are associated with significantly increased risk of NCEIS. Plasma proteomics revealed that PROZ variant carriers also have significantly higher circulating levels of two proteins that are known to play a role in acute ischemic stroke. By contrast, we found no association between PROZ mutations and an increased risk for VTE, MI, PAD, or bleeding. Taken together, these findings suggest that protein Z haploinsufficiency likely confers a uniquely higher risk of ischemic stroke without substantially influencing other forms of thrombosis.
Disclosures
Ellinor:Bayer AG: Consultancy, Research Funding; Novartis: Consultancy. Bendapudi:Takeda Pharmaceuticals: Consultancy; Alexion Pharmaceuticals: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal